Diels Alder Reaction Essay - apologise, but
Get e-Alerts Abstract Although gem-diborylalkenes are known to be among the most valuable reagents in modern organic synthesis, providing a rapid access to a wide array of transformations, including the construction of C—C and C-heteroatom bonds, their use as dienophile-reactive groups has been rare. Herein we report the Diels—Alder DA reaction of unsymmetrical gem-diborylalkenes. These reactions provide a general and efficient method for the stereoselective conversion of gem-diborylalkenes to rapidly access 1,1-bisborylcyclohexenes. Using the same DA reaction manifold with borylated-dienes and gem-diborylalkenes, we also developed a concise, highly regioselective synthesis of 1,1,2-tris- and 1,1,3,4-tetrakis boronates cyclohexenes, a family of compounds that currently lack efficient synthetic access. Furthermore, DFT calculations provided insight into the underlying factors that control the chemo-, regio-, and stereoselectivity of these DA reactions. This method also provides stereodivergent syntheses of gem-diborylnorbornenes.Diels Alder Reaction Essay Video
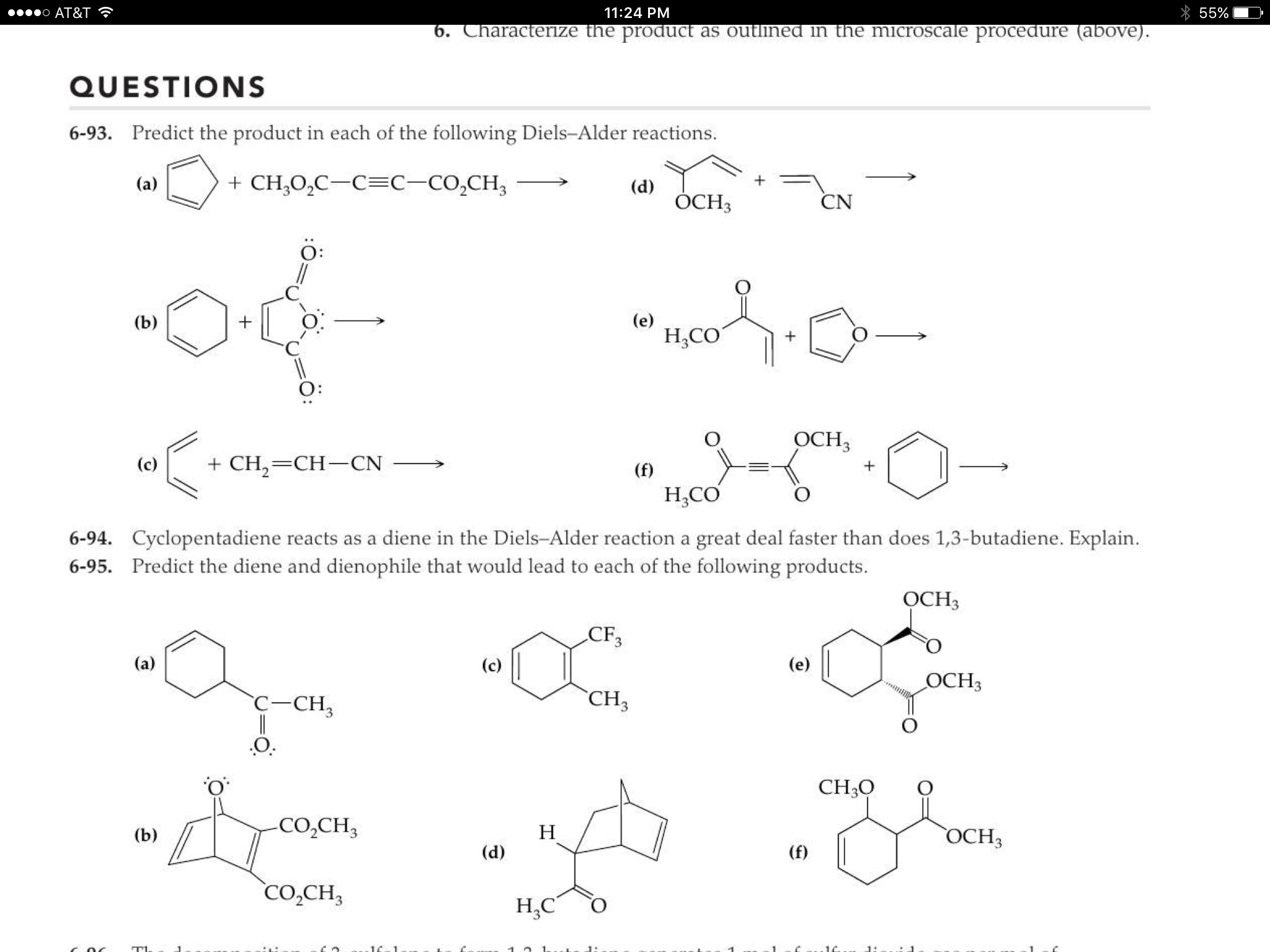
Chem 334: Diels-Alder Reaction Diels Alder Reaction Essay![[BKEYWORD-0-3] Diels Alder Reaction Essay](https://s3.studylib.net/store/data/008286519_1-31a3a516918b2ecb8e4b3f990785fd2b.png)
Synthesis Problems? Find Fast and Better Solutions for Organic Transformations Science of Synthesis is your one-stop tool for full-text reviews of organic transformations and experimental procedures. The online reference work helps speed up your literature research and improve your strategy for the design of new molecules.

Science of Synthesis provides a critical revi Thieme Chemistry is part of the privately owned Thieme Publishing Group. We sell a full range of products: Journals, Reference Works, Encyclopedias]
I am assured, what is it — a false way.
I refuse.