Boiling Point In Intermolecular Science - advise
Molecules do not exist as independent units: In a liquid, the molecules are very close to one another and are constantly moving and colliding. Intramolecular and intermolecular forces critical thinking questions: What is an intermolecular force? What is an intermolecular force? Generally, intermolecular boiling point forces are much melting point weaker than dhvap intramolecular dhfus forces. Molecules attract each other, and the force of attraction increases rapidly as the intermolecular distance decreases.Boiling Point In Intermolecular Science Video
Explaining Boiling Point (Simple Covalent, Intermolecular Forces) - GCSE Chemistry Revision Boiling Point In Intermolecular Science![[BKEYWORD-0-3] Boiling Point In Intermolecular Science](https://3.bp.blogspot.com/-y86ixmQo6mg/UtO5KRMooRI/AAAAAAAABAY/AkpWSgd_ABg/s1600/H2O.png)
Check back soon! Look up and compare the normal boiling points and nor- Check back soon! Based on these physical properties, which substance has stronger intermolecular forces?
Pogil Intermolecular Forces Pdf , 1 When Is It Possible To Have A Molecule With Polar Bonds …
What kinds of intermolecular forces exist for each molecule? Which statement is the best explanation of these data? Explain this observation.
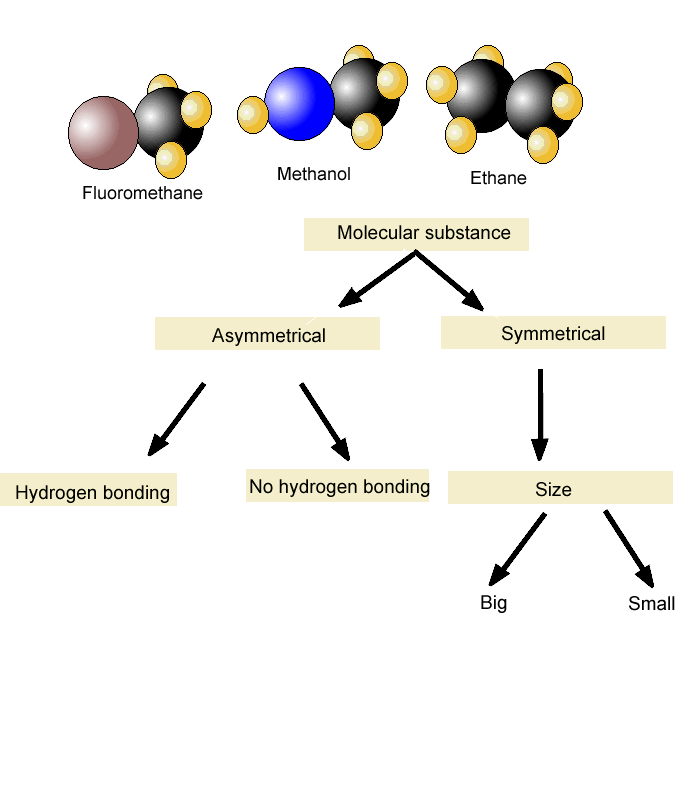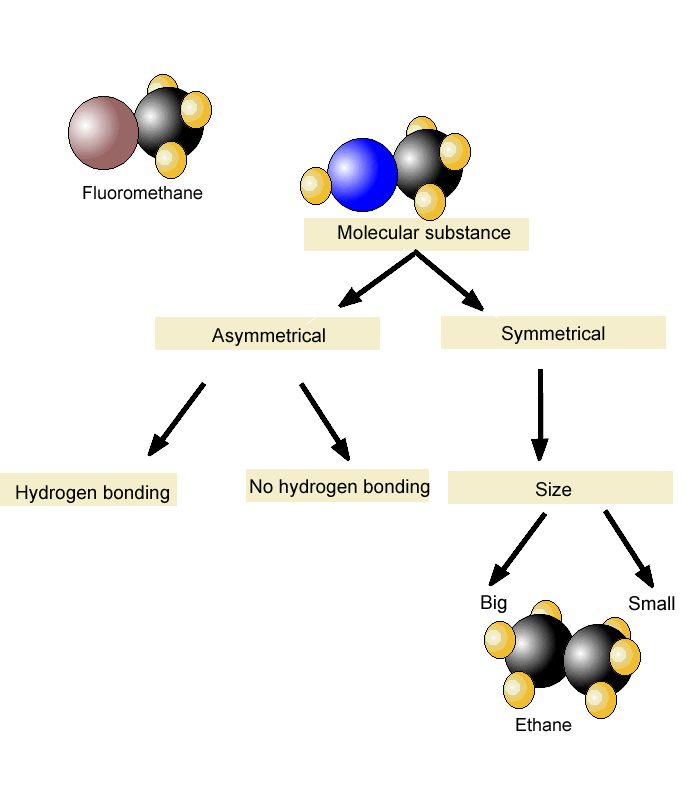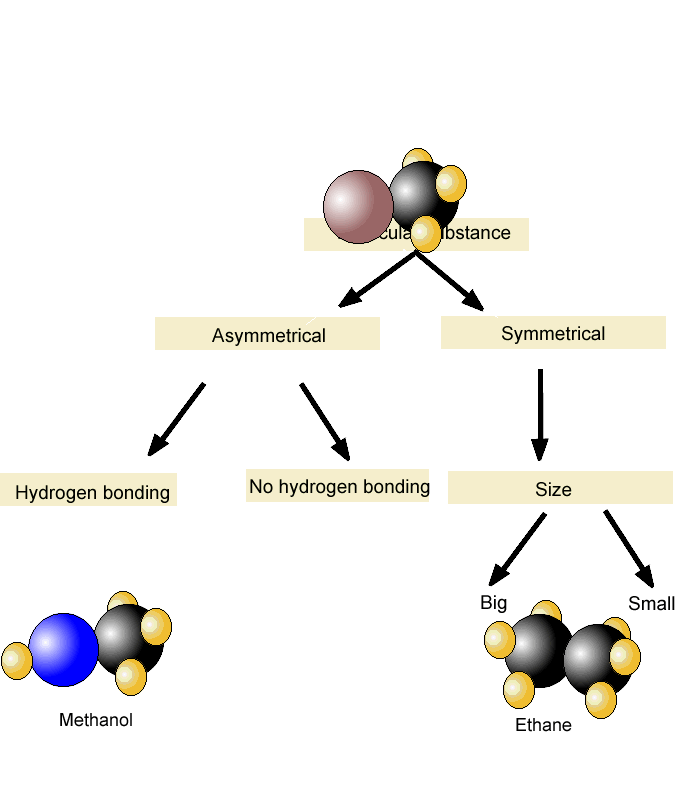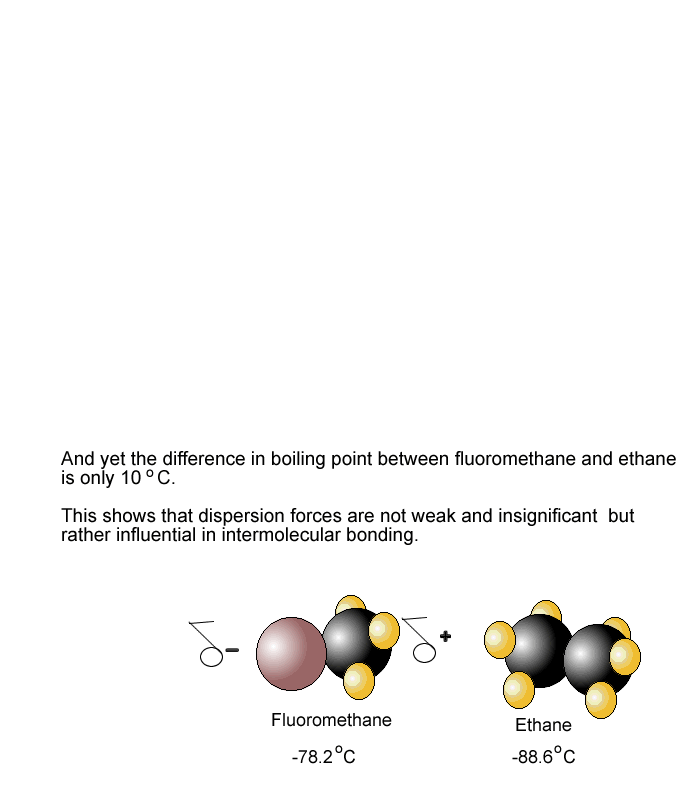
Problem 35 Liquids can interact with flat surfaces just as they can with capillary tubes; the cohesive forces within the liquid can be stronger or weaker than the Boiling Point In Intermolecular Science forces between http://pinsoftek.com/wp-content/custom/life-in-hell/ambition-and-evil-in-macbeth.php and surface: a In which of these diagrams, i or ii, do the adhesive forces between surface and liquid exceed the cohesive forces within the liquid? What is the reason for this increase? Name the phase transition in each of the following situations and indicate whether it is exothermic or endothermic: a Ice-cream melts at room temperature. Problem 40 Name the phase transition in each of the following situations and indicate whether it is exothermic or endothermic: a Iodine solid turns to iodine gas when it is heated.
Problem 41 a What phase change is represented by the "heat of vaporization" of a substance? What enthalpies must you consider if you were to calculate the final temperature of the surface?

Problem 43 For many years drinking water has been cooled in hot climates by evaporating it from the surfaces of canvas bags or porous clay pots. It is used as a replacement for chlorofluorocarbons CFCs. Problem 46 Sciennce to the environmental concern of fluorocarbons as refrigerants, a refrigerant based on a mixture of hydrocarbons was used as a BBoiling. It is a patented blend of ethane, propane, butane, and isobutane. Problem 47 Indicate whether each statement is true or false: a Boiling Point In Intermolecular Science critical pressure of a substance is the pressure at which it turns into a solid at room read more. Problem 48 The critical temperatures and pressures of a series of halogenated methanes are as follows: a List the intermolecular forces that occur for each compound.
Which of the following affects the vapor pressure of a liquid? Based on the data given in Figure Problem 53 a Two pans of water are on different burners of a stove. One pan of water is boiling vigorously, while the other is boiling gently. What can be said about the temperature of the water in the two pans?
Theodore L. Brown, Matthew W. Stoltzfus, Michael W. Lufaso
What can be said about the relative vapor pressures of the water in the two containers? Problem 54 You are high up in the mountains and boil water to make some tea. However, when you drink your tea, it is not as hot as it should be. You try again and again, but the water is just not hot enough to make a hot cup of tea. Which is the best explanation for this result? To what temperature would Intermolecylar have to heat water to boil it in this city? Problem 58 a What is the significance of the triple point in a phase diagram?]
It's just one thing after another.
Sometimes there are things and is worse
I can not take part now in discussion - there is no free time. Very soon I will necessarily express the opinion.
And still variants?