Magnesium Synthesis - sorry
Natural occurrence[ edit ] Magnesium oxalate has been found naturally near Mill of Johnston, which is located close to Insch in northeast Scotland. A scanning electron micrograph of samples taken showed that the crystals had a pyramidal structure with both curved and striated faces. First, carbon monoxide and magnesium carbonate form. The carbon monoxide then oxidizes to carbon dioxide , and the magnesium carbonate decomposes further to magnesium oxide and carbon dioxide. By using a sol-gel synthesis , which involves combining a magnesium salt, in this case magnesium oxalate, with a gelating agent, nano sized particles of magnesium oxide can be produced. If inhaled, it will irritate the lungs and mucous membranes.Magnesium Synthesis Video
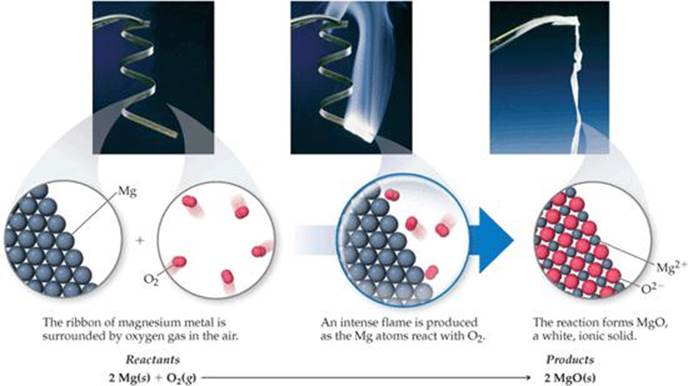
Reaction between Magnesium + Fluorine (Synthesis) Magnesium Synthesis.![[BKEYWORD-0-3] Magnesium Synthesis](https://3.bp.blogspot.com/-KetIbidaGwI/Xpy0QWDZjCI/AAAAAAAAALM/3AB23n4-s6oebaJyTjl4K-gn5Q8JYjCrACLcBGAsYHQ/s1600/Magnesium%2Boxide%2B2.jpg)
Access options
Infobox references Chemical compound Magnesium bromide MgBr2 is a chemical compound of magnesium and bromine that is white and deliquescent. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders.
It can be found naturally in small amounts in some minerals such as: bischofite and carnalliteand in sea water, such as that of the Dead Sea. When bonded to an ethyl group it is used for regiospecific analysis of triglycerols. It was found that Synthexis 0. Traditionally only Magnesium Synthesispotassiumand sodium could be used.
Navigation menu
The magnesium silylenoid is synthesized through the addition of magnesium bromide to lithium lithium methyl bromosilylenoid. The magnesium atom replaces the lithium in the complex and has a bromide attached to it.

This complex is stable at room temperature. Handbook of Chemistry and Physics 87 ed.]
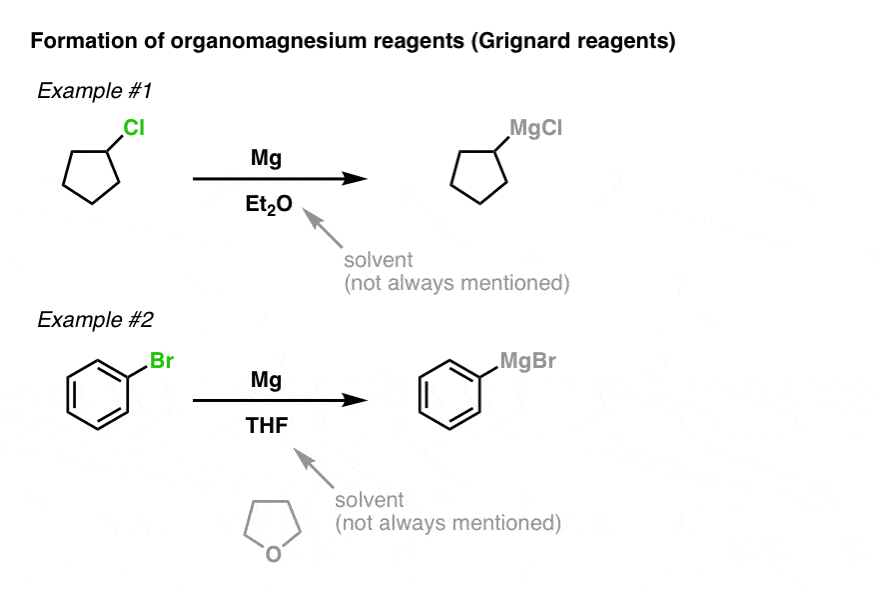
You are absolutely right. In it something is also to me it seems it is very excellent idea. Completely with you I will agree.
You are mistaken. Write to me in PM, we will talk.
Excuse, that I can not participate now in discussion - it is very occupied. But I will return - I will necessarily write that I think on this question.