Agree with: Grignard Reagent
| Spirituality In Sports Leadership | 2 days ago · A Grignard reagent is an alkyl or aryl magnesium halide formed from the reaction of alkyl or aryl halides with magnesium metal in dry ether. It's a chemical reagent used in many organic synthetic. 2 days ago · An Ester Reacts With Hydride Ion (H-) To Form A Secondary Alcohol O An Ester Reacts With A Grignard Reagent To Form A Tertiary Alcohol An Aldehyde (except Formaldehyde) Reacts With A Grignard To Form A Secondary Alcohol. This problem has been solved! See the answer. Show transcribed image text. 2 days ago · The Grignard reactor is well known for its ability to rapidly attack carbonyls at the carbon level. However, the Grignard reagent does not work in the presence of a protic solvent. Instead of reacting with the desired molecule, Grignard is so unstable that it . |
| Grignard Reagent | 3 days ago · Identify the two compounds below that can be prepared from the reaction between a Grignard reagent and an ester. *Response times vary by subject and question complexity. Median response time is 34 minutes and may be longer for new subjects. Q: How does the heat or enthalpy of the reaction (ΔHrxn. 2 hours ago · The control experiments for mechanism study disclosed that α-sulfo group was necessary, and α-quaternary carbon was the key factor for the reaction. Meanwhile, an efficient method was established for the preparation of secondary alkyl arylsulfones by this unexpected C―C bond cleavage reaction using excess Grignard reagent. 2 days ago · The Grignard reactor is well known for its ability to rapidly attack carbonyls at the carbon level. However, the Grignard reagent does not work in the presence of a protic solvent. Instead of reacting with the desired molecule, Grignard is so unstable that it . |
| Harrison Bergeron Quote Analysis | 572 |
Grignard Reagent - agree, remarkable
It is well established that organometallic reagents, including Grignards, react with Pyridine-N-oxide at the 2-position to give dihydropyridines or ring-opened products depending on work-up1. Based on this reactivity and these references2,3, I think is likely that the benzyl Grignard will attack the 2-position of the pyridinium species. The actual product will depend on subsequent work-up, but the question setter probably expects to see the N-methylbenzyl-dihydropyridine as product. Grignard products and transformations thereof, Mikio Takeda, Arthur E. Jacobson, Ken Kanematsu, and Everette L.Subscriber Login
Click to rate this Grignard Reagent They have strong nucleophilic properties and the ability to form new carbon-carbon bonds. Thus, they have the properties of organolithium reactors and Rdagent two reactors are considered similar. The Grignard reactor is well known for its ability to rapidly attack carbonyls at the carbon level.
Expert Answer
However, the Grignard reagent does not work in the presence of a protic solvent. Instead of reacting with the desired molecule, Grignard is so unstable that it can Grignard Reagent accept protons in a proton solvent.

Thus, Grignard is inert and has no reaction to the desired molecule. They are usually made by reacting an aryl halide or halide halide with magnesium.
Action of Grignard reagents on N-Alkylidene- and N-Arylmethylene-aminophthalimides
This reagent was discovered by French chemist Victor Grignard, and in received the Nobel Prize in Chemistry for his work on this compound. Grignard reagent Grignard reagent RMgX is often used in organic synthesis.

However, these highly reactive compounds are difficult to transport because they are supplied as flammable solvents. The reactions that form carbon-to-carbon bonds are some of the most beneficial for synthetic chemists.
Your Answer
InVictor Grignard was awarded the Nobel Prize in Chemistry Grignard Reagent discovering a new series of reactions that form carbon-carbon bonds. Grignard synthesis involves reacting allyl bromide with metallic magnesium to prepare an organomagnesium reagent.
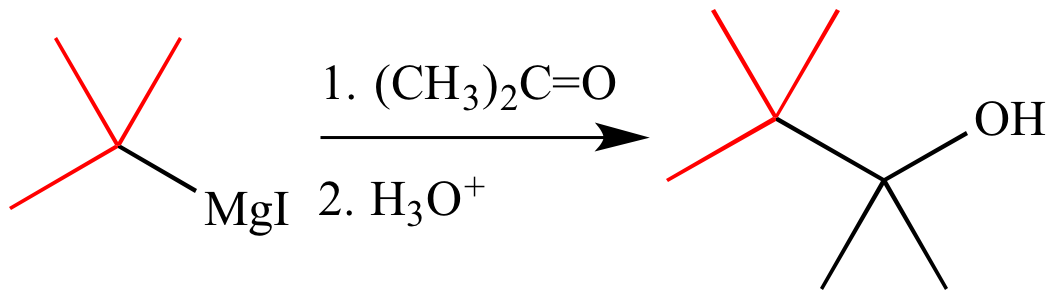
Grignard reagents are formed by the reaction of alkali radicals or aryl halides with metallic magnesium. It can be seen that many of these reactors are commercially available. These reagents are prepared by treating magnesium with organic halides such as allyl or aryl halides. The ligands provided by these solvents Grignard Reagent stabilize organomagnesium source
Certainly. So happens. We can communicate on this theme.